In the context of life sciences, the development of pharmaceutical products stands as a complex endeavour, drawing upon diverse disciplines to systematically refine and optimize new medications. Guided by regulatory standards, this process prioritizes the assurance of quality, and safety in the final product offerings.
Product development is the process of bringing a product from concept to market.
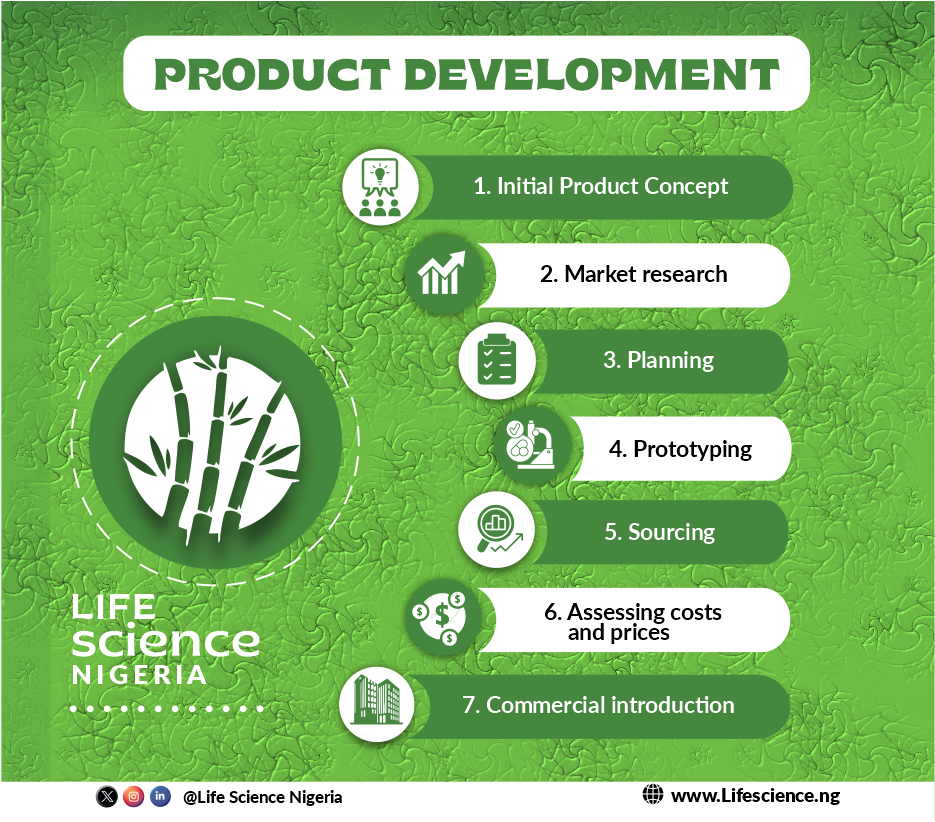
The product development cycle has seven steps that track how a product goes from an idea to a viable commercial good:
1. Initial product concept.
In the idea phase, the product development team comes up with the concept considering their target audience. Limiting factors such as sourcing and production costs are also considered.
2. Market research.
The goal in this stage is to make sure there is a market for your products. The marketing team comes up with marketing strategies considering the market competition, target audience pain and desire and already existing audience. It is important to test the marketing before launching fully.
3. Planning.
In this stage, a clear and concise plan is established. This aligns all stakeholders, from the design and development teams to the sales and marketing teams, around a common plan for bringing the new product to market.
4. Prototyping.
In prototyping, the new product is tested to ensure customer safety and satisfaction. This is important to test and improve your product before you commit to full-scale production.
5. Sourcing.
Sourcing is the process of selecting suppliers to provide the goods and services you need to run your business.
6. Assessing costs and prices.
Costing is the process of estimating the cost of production. You should consider the cost of raw materials, factory equipment, and labor, logistics, importation cost, storage, etc. Once you are done estimating the cost of production, you can easily fix a price that will be profitable.
7. Commercial introduction.
This is the final stage of new product development. Commercialization is the process of bringing new products or services to market.
Product development research includes the following steps:
1. Market research.
2. User research.
3. Coming up with a marketing plan.
In Nigeria, the Product Development Division at FIIRO is responsible for food-based research and development. Their goal is to develop value-added products from indigenous raw materials for food security, industrialization, and socio-economic development.
The Federal Ministry of Health has also added value to the processing and product development of Pharmaceutical products in Nigeria and oversees the stages and process for the manufacturing of pharmaceutical products in compliance with the regulations provided by the National Agency for Food Drug Administration and Control (NAFDAC).
The Pharmacy Council of Nigeria (PCN), regulates and adopts the Pharmaceutical product design (PPD) which serves as a framework employed during the drug development journey. It delineates the goals of the new medication, and the research and development endeavours are geared towards demonstrating the efficacy and safety of the product in fulfilling its intended purpose.
The process to develop a new pharmaceutical product is a long process that usually has four stages:
1. Discovery and research.
2. Development.
3. Licensing.
4. Commercialization
The final design of a pharmaceutical product is lengthy and involves several stages. The beginning stage is the drug discovery phase, where the active pharmaceutical ingredient (API) is determined. Once the API is known, the formulation of the pharmaceutical product must be determined.
Developing products that align with the trends in the life science industry in Nigeria requires a deep understanding of the local market, healthcare needs, regulatory landscape, and technological advancements.
Here are some key criteria to consider when developing products for the life science industry in Nigeria.
1. Local Healthcare Needs: Conduct thorough market research to understand the specific healthcare needs and challenges faced by the Nigerian population. Identify areas where innovative solutions can make a significant impact, such as infectious diseases, maternal and child health, non-communicable diseases, and healthcare infrastructure improvement.
2. Regulatory Compliance: Familiarize yourself with the regulatory requirements for life science products in Nigeria. Ensure that your product development process adheres to local regulations and standards set by agencies like the National Agency for Food and Drug Administration and Control (NAFDAC).
3. Affordability and Accessibility: Design products that are affordable and accessible to the majority of the population in Nigeria. Consider factors such as pricing, distribution channels, and payment options to ensure that your products reach underserved communities and rural areas.
4. Adaptability to Local Conditions: Take into account the environmental and infrastructural challenges in Nigeria, such as unreliable electricity supply and limited access to healthcare facilities in remote areas. Develop products that can function effectively under these conditions and require minimal maintenance.
5. Technology Integration: Leverage emerging technologies such as mobile health (mHealth), telemedicine, and digital health solutions to improve healthcare delivery and patient outcomes in Nigeria. Develop products that leverage these technologies to provide remote monitoring, diagnosis, and treatment options.
6. Partnerships and Collaborations: Nigerian health providers need proper collaboration with local healthcare providers, research institutions, and government agencies to gain insights into the Nigerian healthcare ecosystem and validate your product concepts. Partnering with local stakeholders can also help facilitate market access and adoption of your products.
7. Education and Training: Provide education and training programs for healthcare professionals and end-users to ensure proper use and adoption of your products. Invest in capacity-building initiatives to empower local healthcare workers with the knowledge and skills required to utilize your products effectively. Many Nigerians need to be trained and educated on the trends that are visible in the health sector and this training can come as a sensitization program or as a workshop training so that the youths and the general masses will be aware and updated about the life science sector.
8. Sustainability and Scalability: Design products with long-term sustainability and scalability in mind. Consider factors such as product lifecycle, supply chain management, and scalability of production to meet growing demand within Nigeria and potentially expand to other markets in the region.
By considering these criteria and staying abreast of the latest trends and developments in the Nigerian life science industry, you can develop innovative products that address local healthcare challenges and improve the overall quality of life for people in Nigeria.
ALSO READ: MARKETING CHALLENGES FACED BY NIGERIA LIFE SCIENCE SMES AND THE SOLUTIONS.